Adaptive Capabilities of Two Varieties of Cowpea in Crude Oil-Contaminated and Un-Contaminated Soils
| Received 31 Jul, 2022 |
Accepted 02 Jan, 2023 |
Published 28 Apr, 2023 |
Background and Objective: Cowpea (Vigna unguiculata (L.) Walp., is well-adapted to the Northern parts of Nigeria than the Southern Region especially the Niger Delta Region of Nigeria. The aim of this study was to investigate the adaptive capabilities of cowpea in crude oil-contaminated and non-contaminated soils. Materials and Methods: Two varieties of cowpea namely TVU-31 and Aloka bean were planted in crude oil-contaminated and un-contaminated soils in three replicates using the randomized complete block design for a 50 days period. Data was collected for vegetative vigor, agronomic parameters and root nodulation. The fungal species associated with the roots were isolated using Yeast Extract Mannitol Agar (YEMA) media and later subcultured in Potato Dextrose Agar Medium (PDA) media for identification. Results: The two cowpea varieties, had intermediate vegetative vigor and a gradual increase in the plant height, root length, root width and number of nodules. From the root architecture, it was observed, that the cowpea varieties in the contaminated soil had more adventitious roots with smaller diameters, shallower basal roots with longer, denser root hairs and higher root biomass than the varieties in the un-contaminated soil. Seven fungal species namely Penicillium chrysogenum, Botryodiplodia the obromae, Mucor circinelloides, Aspergillus flavus, Aspergillus spp., Neurospora spp. and Aspergillus niger) were identified to associate with the roots of cowpea. Conclusion: This study, therefore, provides preliminary data for further research into the adaptive capabilities of cowpea in Nigeria.
| Copyright © 2023 Odogwu et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Cowpea (Vigna unguiculata (L.)) Walp, belongs to the family Fabaceae and is known as Southern pea, black eye pea, Crowder pea and Lubia, Niebe, Coupe or Frijole1. It is an important legume crop, widely grown under low-input production systems in arid and semi-arid agro-ecologies of the world2. Cowpea is one of the most ancient crops known to man, in which its origin and domestication occurred in Southern Africa, later, it spread to East and West Africa and Asia3. Now-a-days it is widely adopted and grown throughout the world, however, Africa still predominates in production. In Africa, Nigeria is the largest producer of cowpea grains globally and the annual production is estimated to be about 6.5 million metric tonnes worldwide. Its grains contain high levels of protein (25%), energy, micro- and macro-nutrients while the foliage is an important source of high-quality livestock feed4,5. It is one of the most preferred crops in the farming systems of the majority of resource-poor rural households in Sub-Saharan Africa (SSA)6, because of its ability to enhance soil fertility as a result of its nitrogen-fixing capability and phosphorus-acquiring ability7.
Cowpea is well adapted to arid and hot areas and can produce a yield of one ton of seed and five tons of hay per hectare with as little as 300 mm of rainfall. One of the adaption features of cowpea is the fact that it is often cultivated with other crops as it tolerates shade8. Another adaption mode of cowpea is the ability to form long taproots and excellent drought tolerance mechanisms which are achieved by turning the leaves upwards to stop them from becoming too hot and closing the stomata9,10. Also, another adaptation mode of cowpea is its ability to capture and utilize nutrients such as nitrogen and phosphorus from the top level of soil, through a symbiotic relationship with soil microflora activities11. The microflora is Bradyrhizobiuim spp., which is the specific symbiotic nodular bacteria that enable cowpea to fix nitrogen, while Arbuscular Mycorrhizal Fungi (AMF) especially those belonging to the phylum Glomeromycota, are among the most important plant symbionts that enable cowpea to acquire phosphorus7.
Cowpea is the main staple food in sub-Saharan Africa, particularly in the dry savanna areas of Nigeria such as Adamawa, Bauchi, Benue, Borno, Gombe, Kaduna, Yobe, Niger, Katsina, Kebbi, Sokoto, Taraba and Zamfara12. Than the Southern Region especially the Niger Delta Region of Nigeria. It has a competitive niche in sandy soils, does not tolerate excessively wet conditions and has low productivity in poorly drained soils, which is the case in the Niger Delta Region of Nigeria. This is further compounded by the fact that crude oil-contamination, which predominantly occurs, is a limiting factor on the growth and adaptation of the plants and is a major factor affecting soil microflora activities in the soil in this region12. According to popoola et al.13, an analysis of soil samples collected from some parts of Rivers State in the Niger Delta Region, showed that the nitrogen content in the soil increased from 0.22% in un-contaminated soil to 2.62% in contaminated soil, while phosphorus content in the soil decreased from 1.53% in un-contaminated soil to 0.38% in contaminated soil. This indicated that crude oil-contamination increased nitrogen and bacteria contents and decreased the amount of phosphorus in the soil in this region. However, with the current call for diversifying crop productivity because of insecurity in the Northern parts of Nigeria and climate change, it will be pertinent to study the adaptive capacity of cowpea in the agro-ecological region of the Niger Delta Region of Nigeria. Very few studies have been carried out on the adaptive capacity of cowpea and the Arbuscular Mycorrhiza Fungi (AMF) linked to phosphorus acquisition in this part of Nigeria.
The aims of the study, therefore, were to investigate the adaptive capabilities of cowpea in crude oil-contaminated and non-contaminated soils, with a view to evaluating their agronomic characteristics, root architecture, nodulation and nodules occupancy by Arbuscular Mycorrhiza Fungi (AMF) and their identification.
MATERIALS AND METHODS
Sources of plant materials: The seeds of two varieties of cowpea, namely Aloka beans and TVU-31, were obtained from the Choba market (4.8886°N, 6.9007°E) in August, 2021 and the germplasm of the International Institute of Tropical Agriculture (IITA), Ibadan, respectively. Aloka beans are a commonly eaten variety readily available and sold in the market. While TVU-31 is a breeder line from IITA.
Experimental site and experimental design: Both the field and laboratory experiments were carried out at the Center for Ecological Studies and the Pathology/Mycology Laboratories in the Department of Plant Science and Biotechnology, University of Port Harcourt, Choba, Rivers State. The experiment was arranged in a randomized complete block design made up of 4-rows with three replications of each cultivar for contaminated and non-contaminated soil samples. The soil used for the study had been used for cultivating cowpea in the previous planting season and had no previous exposure to crude oil. The crude oil used for this study was obtained from Shell Petroleum Development Company of Nigeria Limited (SPDC) Agbada flow station, Igwuruta, Rivers State, Nigeria (142°4055'N, 55.951°700'E). Ten grams of soil each were put in each twenty 5 L buckets, with 10 of the buckets treated (or contaminated) with 0.08 mL of crude oil and the remaining 10 buckets not treated (not contaminated) with crude oil. The treated soils were allowed to sit for two weeks before planting the seeds. After which, four seeds of each variety were planted in each bucket and allowed to germinate.
Agronomic assessment, root architecture and nodules assessment: After germination, agronomic assessments, root architecture and nodulation assessment data were collected every 10 days for a period of 50 days7. The plants were harvested and the roots were carefully washed to remove soil debris before further observations were made. The plant height, shoot, root length and root width were measured using a 30 cm meter ruler. The plant weight, shoot and root weights were measured with a Stuccler digital weighing balance (Putian, Fujian Province, China). The number of leaves and nodules were manually counted while the rating of the vegetable vigour was determined with visual observation of the plant parts. The visual observations of the root architecture and morphology with subsequent changes were noted and categorized based on the report by Ngkou et al.7. For the vegetative adaptation, the evaluation was based on the effect the growth habit has on the plant vigour and rated on a scale of 1 to 9, where, 1: Excellent, 3: Good, 5: Intermediate, 7: Poor and 9: Very poor. To assess the nodulation, the number of effective-appearing nodules with red to pink internal colour is determined and the number of nodules per plant was rated on a scale of 1 to 9 where, 1: Excellent (with more than 80 nodules), 3: Good (with 41-80 nodules), 5: Intermediate (with 21-40 nodules), 7: Poor (with 10-20 nodules) and 9: Very poor (with less than 10 nodules). All data obtained were subjected to statistical analysis using Microsoft Excel (2016) and presented as bar charts.
Determination of nodules occupancy by Arbuscular Mycorrhiza Fungi (AMF): For determination of nodules occupancy by Arbuscular Mycorrhiza Fungi. The roots of the plant were thoroughly washed under tap water to remove the soil debris and transferred into sterile water and kept overnight in the refrigerator at 40°C. The following day, the nodules were removed from the refrigerator and soaked for an hour in distilled water at room temperature. Rehydrated nodules are sterilized using sodium hypochlorite. All nodules morphotypes are subjected to 95% alcohol for 30 sec, rinsed 6 times in sterilized distilled water and then in sodium hypochlorite again for 5 min followed by 6 rinses with several changes of distilled water. Healthy and pink nodules were selected for the isolation of nodule-associated fungi.
Characterization and identification of the fungi associated with the nodule: The nodules were then selected and crushed on a sterile petri dish using sterile forceps. Then the contents were streaked into already prepared Yeast Extract Mannitol Agar (YEMA) medium (made up of 10 g mannitol, 0.5 g of KH2PO4 or K2HPO4, 0.1g of NaCl, 1 L of distilled water, 15 g of agar pH = 6.8) and were repeated three times. The Petri dishes with nodules were then incubated at room temperature for 3 days in an inverted position and observed. Three days after incubation, all streaked Petri dishes are observed and all colonies that are formed were counted and checked for their colony morphologies. Fungal colonies with distinct phenotypic characteristics were isolated aseptically and subcultured on Potato Dextrose Agar Medium (PDA) at room temperature for three days. Morphological identification and characterization were carried out according to Sampson et al.14.
Statistical analysis: The growth studies data (plant height, shoot and root length, root width, plant weight, shoot and root weights, number of leaves and number of nodules) obtained from this study were subjected to statistical analysis using Microsoft Excel (2016) and presented as bar charts.
RESULTS
The vegetative adaptation of the varieties of cowpea used in this study was presented in Fig. 1-4. From the result, it was observed that all plants in both the contaminated and un-contaminated soils had an intermediate (5) vegetative vigor at 10 days after germination and good (3) vegetative vigor from 20 to 50 days after germination.
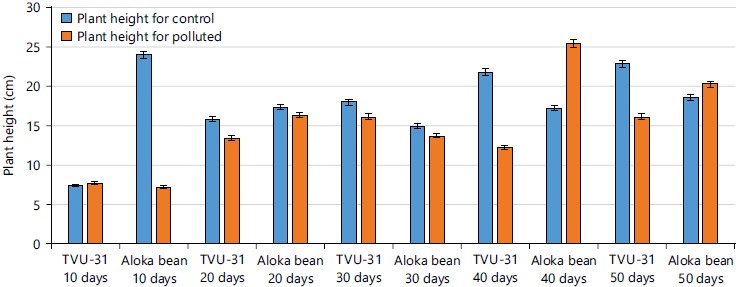
Agronomic characteristics after 10 to 50 days of germination: The plant height of the two varieties of cowpea plants from 10 to 50 days after germination in contaminated and un-contaminated crude oil soils were shown in Fig. 1. From the result it was observed that the TVU-31 variety plant heights ranged from 7.4-22.8 cm for un-contaminated soil and 7.2-16.2 cm in the contaminated soil while Aloka bean variety had plant heights range of 7.2-25.5 cm for un-contaminated and 11.2-18.6 cm for polluted soil for the 10 and 50th days, respectively after germination. Results showed that plant height was affected by the polluted soil in both cowpea varieties.
|
|

|
|
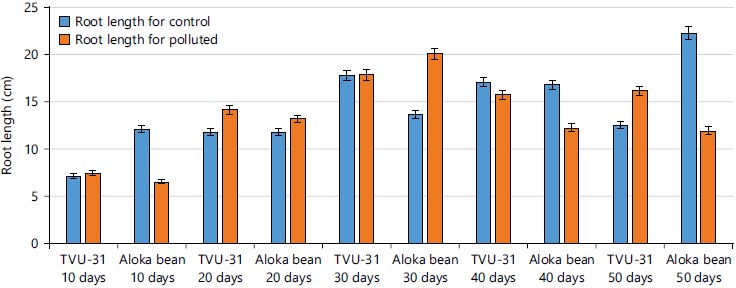
The result of the root length of the two varieties of cowpea plants from 10 to 50 days after germination in contaminated and un-contaminated crude oil soils was shown in Fig. 2. From the result, it was observed that the length of the roots of TVU-31 ranged from 7.4-17.8 cm and 7.1-17.8 cm for both contaminated and un-contaminated, respectively, while Aloka bean root length ranged from 6.5-20.3 and 12.1-22.3 cm for both contaminated and un-contaminated, respectively. However, it was observed that the highest value for the root length in both varieties was recorded on the 30th day after germination.
The result of the root width of the two varieties of cowpea plants from 10 to 50 days after germination in contaminated and un-contaminated crude oil soils were shown in Fig. 3. From the results, it was observed that the width of the roots of TVU-31 ranged from 9.3-25.3 and 9.1-27.8 cm for both un-contaminated and contaminated soils, respectively, while Aloka bean root width ranged from 5.1-23.7 and 13.2-25.5 cm and for both contaminated and un-contaminated, respectively. Also, it was observed that the Aloka bean generally had the highest root width value for both contaminated and un-contaminated crude oil soils on the 50th day while TVU-31 recorded its highest value on the 40th day after germination.
|

|
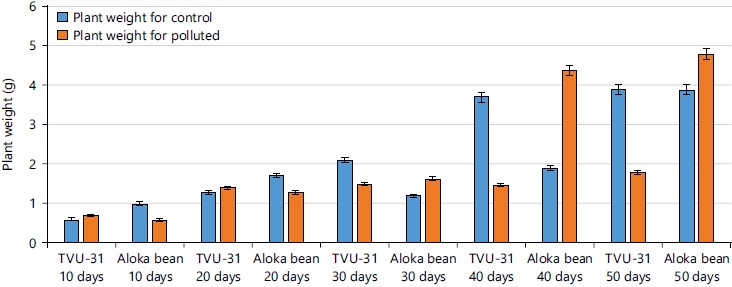
The result of the plant weight of the two varieties of cowpea from 10 to 50 days after germination in un-contaminated and contaminated crude oil soils was shown in Fig. 4. From the results, it was observed that the width of the roots of TVU-31 ranged from 0.6-3.7 and 0.7-1.8 g for both un-contaminated and contaminated soils respectively, while Aloka bean root width ranged from 1.0-3.9 and 0.6-4.8 g for both contaminated and un-contaminated, respectively. Also, it was observed that there was a gradual increase both TVU-31 and Aloka bean varieties recorded the highest weight on day 50 for both polluted and control while the lowest weight was recorded on the 10th day after germination.
Root architecture, morphology and nodulation: The results of the root architecture, morphology and nodulation were shown in Plate 1a-e. From Plate 1a and b, it was observed that the root architecture and morphology for the roots in both the un-contaminated and contaminated soils from the 10 to 50 days period in both cowpea varieties was observed to be gradual and similar changes occurred on, b, both varieties. At the 10th day old, the roots had some adventitious roots with shallow basal roots, while the 50th day old roots had more adventitious roots that were smaller in diameter and had shallower basal roots with more dispersed laterals. There was greater root biomass with longer and denser root hairs.
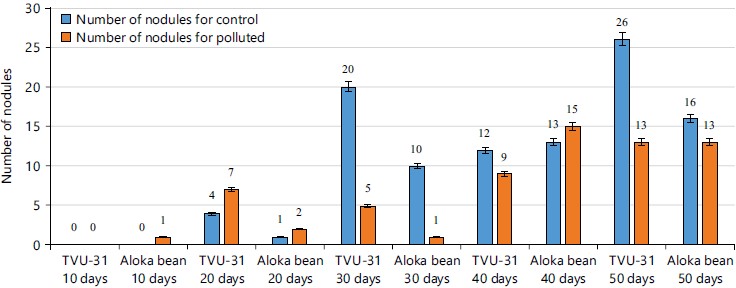
The result of the root nodulation and architecture of the two varieties of cowpea from 10 to 50 days after germination in the un-contaminated and contaminated crude oil soils were shown in Fig. 5. From the result, it was observed that one nodule appeared in the Aloka bean after 10 days of germination in the contaminated soil, while four nodules were seen in TVU-31 roots in the un-contaminated soil 20 days after germination. The highest number of nodules was observed in TUV-31 (26 nodules) grown in un-contaminated soil after 50 days of germination, while the highest number of nodules in Aloka bean (15 nodules) was observed 40 days after germination in contaminated soil.
Morphological identification of fungi associated with the nodule: The fungi associated with the root nodules of cowpea morphologically identified were Penicillium chrysogenumin, Aspergillus flavus, Mucor circinelloides and Botryodiplodia theobromae.
DISCUSSION
This study evaluated the adaptive capabilities of two varieties of cowpea namely TVU-31 and Aloka bean in crude oil-contaminated and un-contaminated soils. From the results of the vegetative vigor, all the cowpea plants in both the contaminated and un-contaminated soils grew till 50 days with intermediate vegetative vigor. This indicated that the cowpea varieties, TVU-31 and Aloka bean has the ability to adapt to crude oil-contaminated soils. Also, from the agronomic features, it was observed that there was a gradual increase in the plant height, shoot and root length, root width, plant and the number of nodules according to the number of days after germination. From the root architecture, it was observed, that the cowpea varieties in the contaminated soil on the 50th day after germination, had more adventitious roots with smaller diameters, shallower basal roots with longer, denser root hairs and higher root biomass than the varieties in the un-contaminated soil. This indicated that the cowpea varieties are adapted to soils with low nitrogen and phosphorus15.
Cowpeas have been reported to be well-adapted to a wide range of soil and climatic conditions16. According to Lynch and Brown15 the adaptation features that allow legumes to survive in a new environment are their vegetative adaptation, agronomic (vegetative) features, root architecture and nodulations. However, changes in the root architecture, morphology and anatomy associated with adaptation indicate low nitrogen and phosphorus in soil.
Legumes generally produce nitrogen-fixing root or stem nodules which form a symbiotic association with Rhizobia and AMF. The presence of arbuscular mycorrhiza is a significant indication of mutualism between plants and AM fungi and is widespread and abundant17. They are formed by bryophytes, pteridophytes, gymnosperms and angiosperms and are ubiquitous in most temperate and tropical ecosystems including agricultural systems. According to Sui et al.18, legumes have a good mycotrophic status and arbuscular mycorrhizas are found in their roots. The fungal partners in AM associations are remarkably abundant, accounting for 5-50% of the microbial biomass in agricultural soils, with the fungal phylum, Glomeromycota being the most common19. From this study, there was a high rate of mycorrhizal colonization of the roots of cowpea. However, the fungi associated with the nodules of cowpea varieties were Penicillium chrysogenumin, Aspergillus flavus, Mucor circinelloides and Botryodiplodia theobromae. According to Doilom et al.20 Increasing the bioavailability of phosphorus in soils for plants, Phosphate-Solubilizing Fungi (PSF) play an important role. The fungal strains of Penicillium and Aspergillus are known as Phosphate-Solubilizing Fungi (PSF). They are fungi that offer a biologically tolerable mean to convert insoluble phosphate to soluble forms, making them accessible for the plant to absorb. Insoluble phosphates are modified into accessible forms using phosphate solubilizing microorganisms through the process of chelation, exchange reactions acidification and production of organic acid. Applying PSMs as bioconverters or biofertilizers to solubilize stable phosphorus has not been effectively practiced even though phosphorus is seen as a restrictive factor in numerous soils21.
However, Dewan et al.10 have reported that the fungus Penicillium chrysogenum in can be used as a biofertilizer for the growth and productivity of certain crops. This study, therefore, paves way for further research into the adaptation capabilities of cowpea in Nigeria.
CONCLUSION
The vegetative adaptation of the two varieties of cowpea, TVU-31 and Aloka bean, used in this study in both the contaminated and un-contaminated soils had intermediate (5) vegetative vigour at 10-days after germination and good (3) vegetative vigour from 20 to 50 days after germination. The agronomic characteristics, that is the plant height, root length, root width and plant weight, were observed to gradually increase in both varieties in both the un-contaminated and contaminated soils. The result of the root nodulation and architecture of the two varieties of cowpea from 10 to 50 days after germination in the un-contaminated and contaminated crude oil soils showed that the highest number of nodules was observed in TUV-31 (26 nodules) grown in un-contaminated soil after 50 days of germination, while the highest number of nodules in Aloka bean (15 nodules) was observed 40 days after germination in contaminated soil. The fungi associated with the root nodules of cowpea morphologically identified were Penicillium chrysogenum in, Aspergillus flavus, Mucor circinelloides and Botryodiplodia theobromae. However, further studies are need to verify the the fungi associated with leguminous plants and their plant-pathogen interactions.
SIGNIFICANCE STATEMENT
This study makes some important contributions to the growth of cowpea in the Southern Region of Nigeria with the knowledge that it is well-adapted to the Northern Region of Nigeria than the Southern Region. Again, with the predominant occurrence of crude oil-contamination in the South, which is a limiting factor on the growth and adaptation of the plants and a major factor affecting soil microflora activities in the soil in this region. We argue that this study moves the field forward because cowpea showed some levels of tolerance hence, they can be recommended to farmers in oil-polluted areas. Therefore, with the current call for diversifying crop productivity because of insecurity in the Northern parts of Nigeria and climate change, it is pertinent to study the adaptive capacity of cowpea in the agroecological region of the Niger Delta Region of Nigeria.
ACKNOWLEDGMENT
The authors would like to acknowledge Mr. John Ogazie for his technical support during the work.
REFERENCES
- Agbogidi, O.M., 2010. Screening six cultivars of cowpea (Vignia unguiculata (L.) Walp for adaptation to soil contaminated with spent engine oil. Acad. Arena, 2: 65-75.
- Horn, L.N. and H. Shimelis, 2020. Production constraints and breeding approaches for cowpea improvement for drought prone agro-ecologies in Sub-Saharan Africa. Ann. Agric. Sci., 65: 83-91.
- Abadassi, J., 2015. Cowpea (Vigna unguiculata (L.) Walp.) agronomic traits needed in tropical zone. Int. J. Pure Appl. Biosci., 3: 158-165.
- Boukar, O., C.A. Fatokun, B.L. Huynh, P.A. Roberts and T.J. Close, 2016. Genomic tools in cowpea breeding programs: Status and perspectives. Front. Plant Sci., 7: 00757.
- Ibro, G., M.C. Sorgho, A.A. Idris, B. Moussa, D. Baributsa and J. Lowenberg-DeBoer, 2014. Adoption of cowpea hermetic storage by women in Nigeria, Niger and Burkina Faso. J. Stored Prod. Res., 58: 87-96.
- Molosiwa, O.O., C. Gwafila, J. Makore and S.M. Chite, 2016. Phenotypic variation in cowpea (Vigna unguiculata [L.] Walp.) germplasm collection from Botswana. Int. J. Biodivers. Conserv., 8: 153-163.
- Ngakou, A., M. Tamò, I.A. Parh, D. Nwaga, N.N. Ntonifor, S. Korie and C.L.N. Nebane, 2008. Management of cowpea flower thrips, Megalurothrips sjostedti (Thysanoptera, Thripidae), in Cameroon. Crop Prot., 27: 481-488.
- Ddamulira, G., C.A.F. Santos, P. Obuo, M. Alanyo and C.K. Lwanga, 2015. Grain yield and protein content of Brazilian cowpea genotypes under diverse Ugandan environments. Am. J. Plant Sci., 6: 2074-2084.
- OECD, 2019. Cowpea (Vigna unguiculata). In: Safety Assessment of Foods and Feeds Derived from Transgenic Crops, OECD (Ed.), Organisation for Economic Cooperation and Development (OECD), ISBN: 987-92-64-79779-6, Paris, France.
- Dewan, M.M., K.F. Alwan and F.H. Alsahaf, 2018. Effects of Aspergillus niger and Penicillium chrysogenum as biofertilizers on growth and productivity of wheat crop. Plant Arch., 18: 1993-1998.
- Omomowo, O.I. and O.O. Babalola, 2021. Constraints and prospects of improving cowpea productivity to ensure food, nutritional security and environmental sustainability. Front. Plant Sci., 12: 751731.
- Omoigui, L.O., A.Y. Kamara, N. Kamai, F. Ekeleme and K.T. Aliyu, 2020. Guide to Cowpea Production in Northern Nigeria. IITA, Ibadan, Nigeria, ISBN: 978-978-131-368-4, Pages: 57.
- Popoola, L.T., A.S. Yusuff, A.A. Adeyi and O.O. Omotara, 2022. Bioaugmentation and biostimulation of crude oil contaminated soil: Process parameters influence. South Afr. J. Chem. Eng., 39: 12-18.
- Samson, R.A., J. Houbraken, U. Thrane, J.C. Frisvad and B. Andersen, 2019. Food and Indoor Fungi. CBS Laboratory Manual Series, United States, Pages: 481.
- Lynch, J.P. and K.M. Brown, 2008. Root Strategies for Phosphorus Acquisition. In: The Ecophysiology of Plant-Phosphorus Interactions, White, P.J. and J.P. Hammond (Eds.), Springer, Netherlands, ISBN: 978-1-4020-8435-5, pp: 83-116.
- Omoigui, L.O., A.Y. Kamara, J. Batieno, T. Iorlamen and Z. Kouyate et al., 2018. Guide to Cowpea Production in West Africa. IITA, Ibadan, Nigeria, ISBN: 978-978-131-357-8, Pages: 60.
- Plenchette, C., C. Clermont-Dauphin, J.M. Meynard and J.A. Fortin, 2005. Managing arbuscular mycorrhizal fungi in cropping systems. Can. J. Plant Sci., 85: 31-40.
- Sui, X., K. Guan, Y. Chen, R. Xue and A. Li, 2022. A legume host benefits more from arbuscular mycorrhizal fungi than a grass host in the presence of a root hemiparasitic plant. Microorganisms, 10: 440.
- Schüβler, A., D. Schwarzott and C. Walker, 2001. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol. Res., 105: 1413-1421.
- Doilom, M., J.W. Guo, R. Phookamsak, P.E. Mortimer and S.C. Karunarathna et al., 2020. Screening of phosphate-solubilizing fungi from air and soil in Yunnan, China: Four novel species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol., 11 :585215.
- Walpola, B.C. and M.H. Yoon, 2012. Prospectus of phosphate solubilizing microorganisms and phosphorus availability in agricultural soils: A review. Afr. J. Microbiol. Res., 6: 6600-6605.
How to Cite this paper?
APA-7 Style
Odogwu,
B.A., Zige,
J.D., Ikechi-Nwogu,
C.G., Emeka,
E.A. (2023). Adaptive Capabilities of Two Varieties of Cowpea in Crude Oil-Contaminated and Un-Contaminated Soils. Research Journal of Botany, 18(1), 9-17. https://doi.org/10.3923/rjb.2023.09.17
ACS Style
Odogwu,
B.A.; Zige,
J.D.; Ikechi-Nwogu,
C.G.; Emeka,
E.A. Adaptive Capabilities of Two Varieties of Cowpea in Crude Oil-Contaminated and Un-Contaminated Soils. Res. J. Bot 2023, 18, 9-17. https://doi.org/10.3923/rjb.2023.09.17
AMA Style
Odogwu
BA, Zige
JD, Ikechi-Nwogu
CG, Emeka
EA. Adaptive Capabilities of Two Varieties of Cowpea in Crude Oil-Contaminated and Un-Contaminated Soils. Research Journal of Botany. 2023; 18(1): 9-17. https://doi.org/10.3923/rjb.2023.09.17
Chicago/Turabian Style
Odogwu, Blessing, Adanta, Josephine Duoye Zige, Chinyerum Gloria Ikechi-Nwogu, and Ethel Anthony Emeka.
2023. "Adaptive Capabilities of Two Varieties of Cowpea in Crude Oil-Contaminated and Un-Contaminated Soils" Research Journal of Botany 18, no. 1: 9-17. https://doi.org/10.3923/rjb.2023.09.17

This work is licensed under a Creative Commons Attribution 4.0 International License.



